Regulatory compliance: How to shape a non-clinical development program and paediatric requirements - YouTube
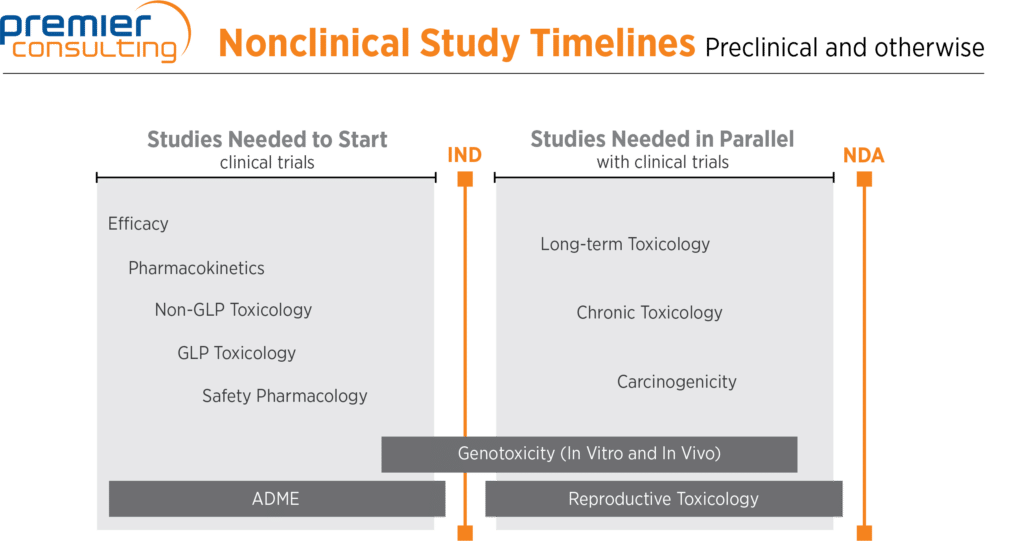
ICH M3 (R2): Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceutica

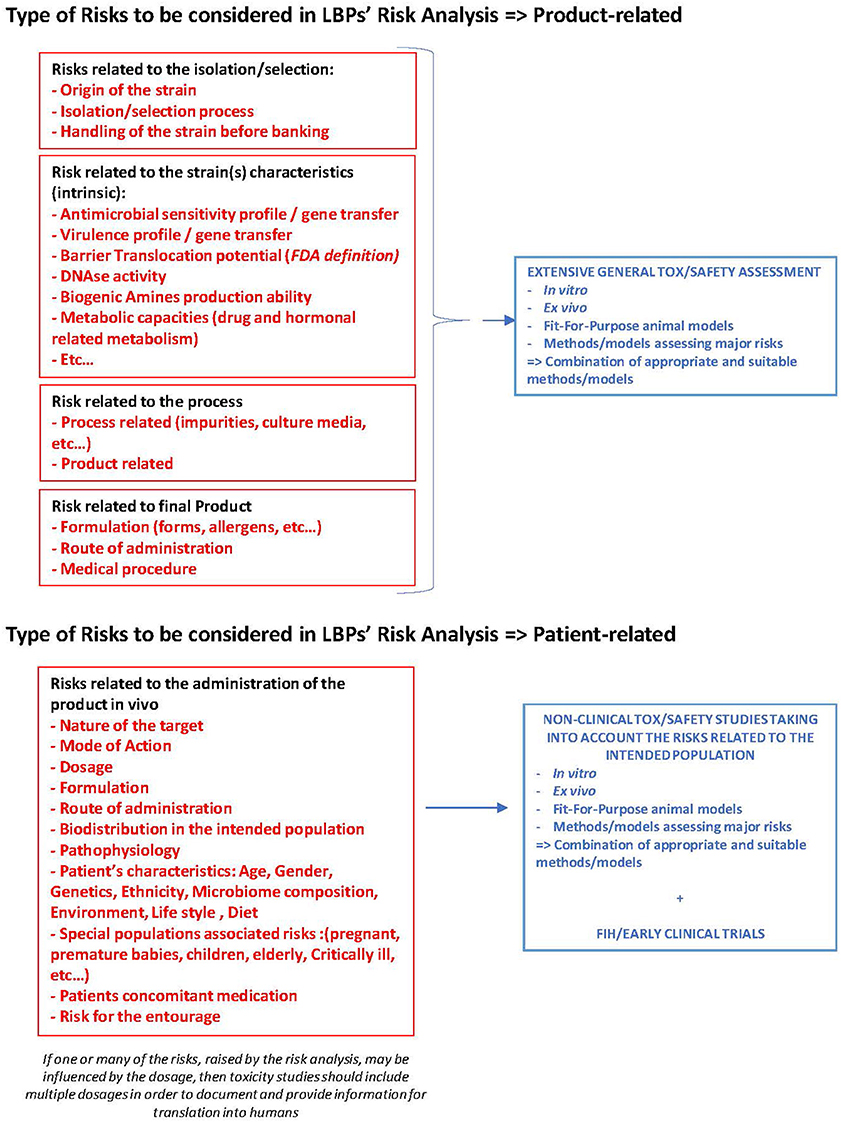
Conclusions from non-clinical safety studies of the OPCs to be injected... | Download Scientific Diagram

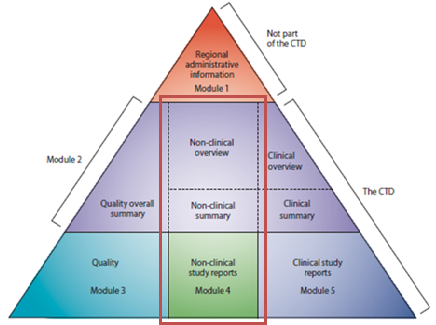
ICH M3(R2) - Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals
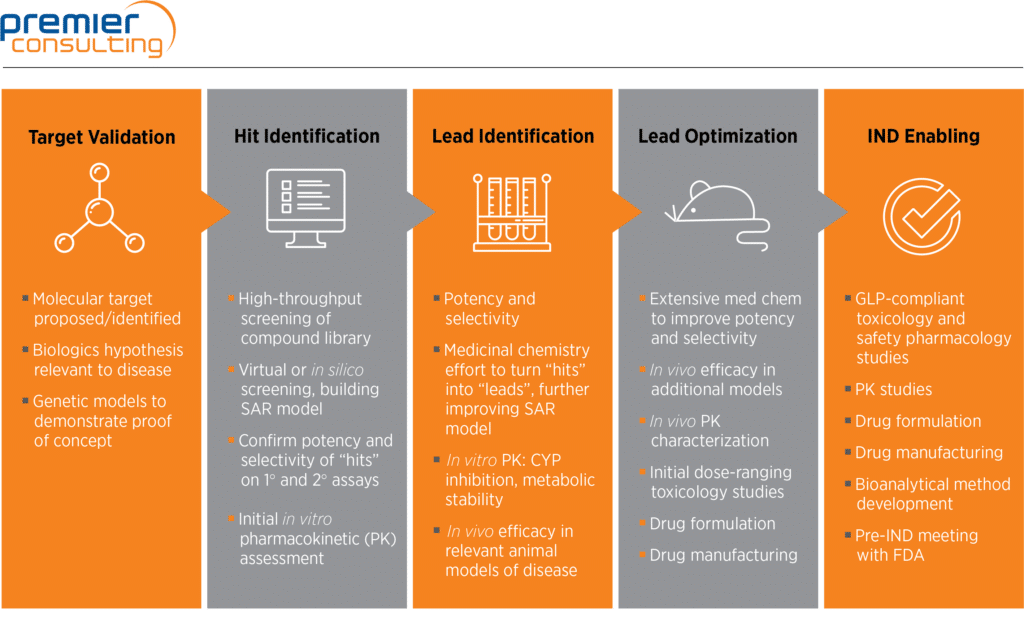
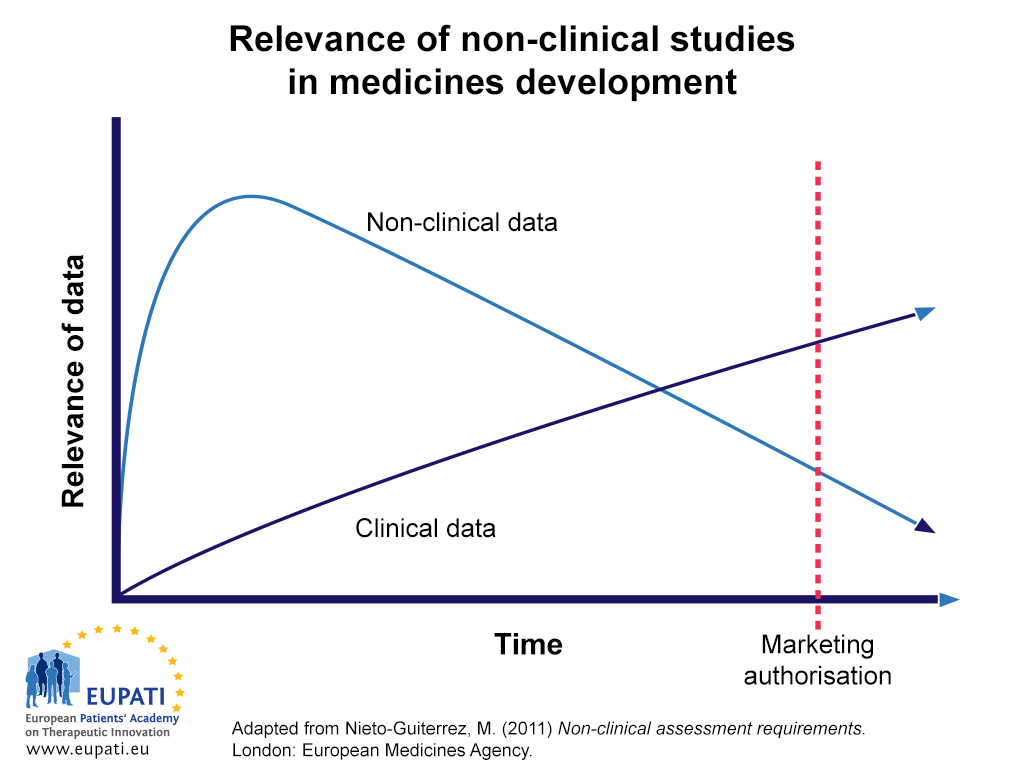
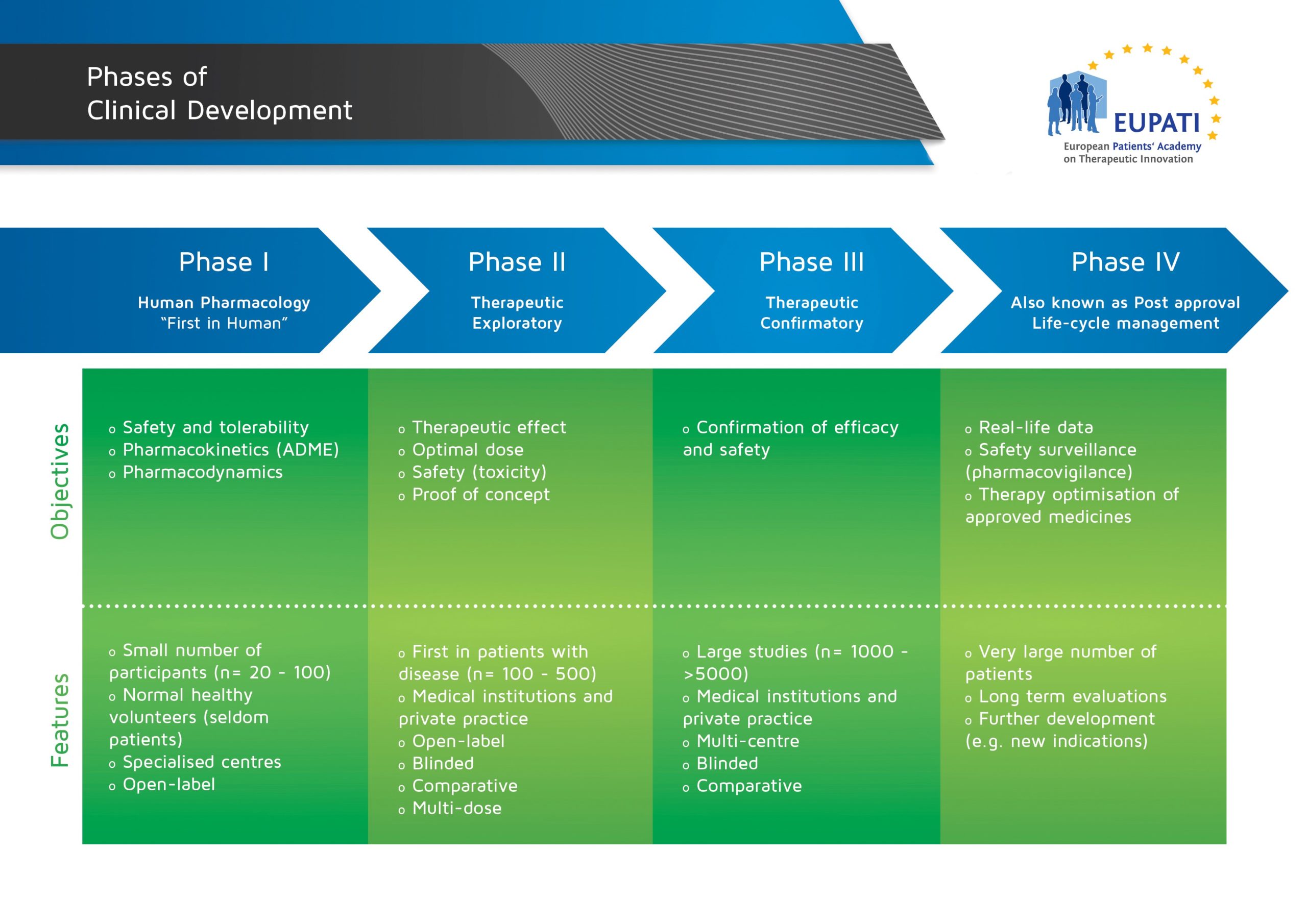
Non-clinical studies in the process of new drug Development--Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies - Document - Gale Academic OneFile

Steps of non-clinical studies in drug development process. GLP: Good... | Download Scientific Diagram
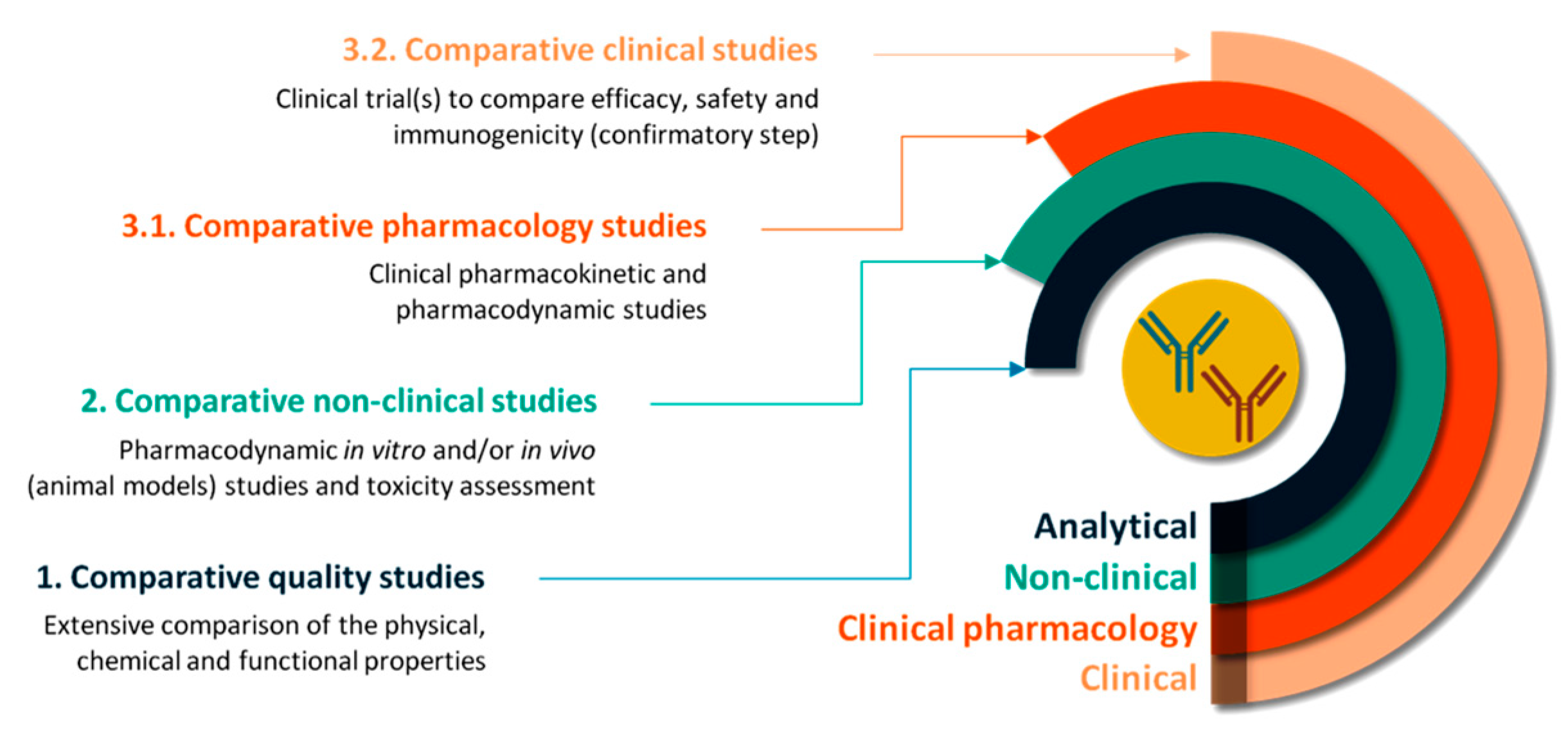
Pharmaceutics | Free Full-Text | Concepts and Challenges of Biosimilars in Breast Cancer: The Emergence of Trastuzumab Biosimilars
Non-clinical safety studies for biotechnologically-derived pharmaceuticals: conclusions from an International Workshop - Susan A Griffiths, Cyndy E Lumley, 1998







![PDF] An introduction to little-known aspects of nonclinical regulatory writing | Semantic Scholar PDF] An introduction to little-known aspects of nonclinical regulatory writing | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3c0319091233e268c650a8558c6a629ecf8655fe/5-Table1-1.png)